New Treatment for Huntington's Disease
#huntington's_disease #medical_treatment #neurodegenerative_disorders

Introduction
In a groundbreaking study, researchers have reported the first successful treatment for Huntington’s disease. This neurodegenerative disorder affects about 1 in 10,000 people worldwide and currently has no cure. The company behind this experimental treatment, Ionis Pharmaceuticals, has released promising data and plans to seek approval from U.S. regulators in the first quarter of next year.
Key Details
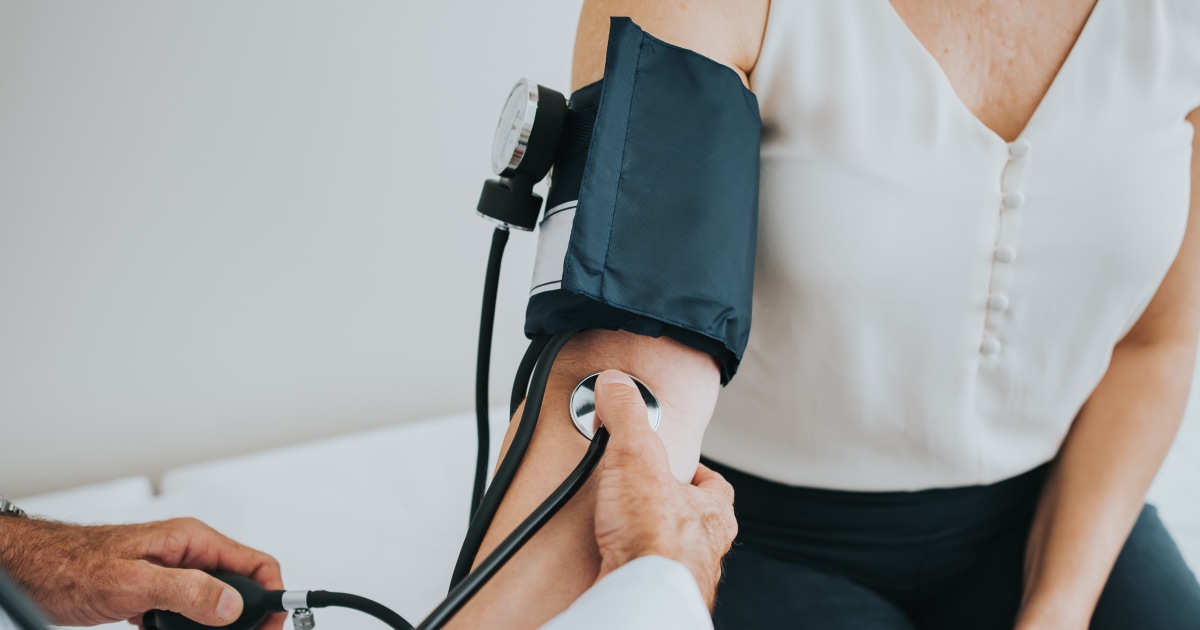
The experimental treatment, called IONIS-HTTRx, works by targeting the genetic mutation that causes Huntington’s disease. In a small study of 46 patients, those who received the highest dose of the treatment showed a significant slowing of disease progression. This is a major breakthrough as previous attempts to treat Huntington’s disease have been unsuccessful.
The study also showed that IONIS-HTTRx was well-tolerated by patients, with no serious side effects reported. This is a crucial factor in seeking approval from regulators and providing hope for those affected by this devastating disease.
Impact
The success of this treatment could have a significant impact on the lives of those with Huntington’s disease. Currently, there is no cure and treatments only focus on managing symptoms. With this new treatment, patients may have the chance to slow or even stop the progression of the disease. It also opens the door for further research and development in the treatment of other genetic disorders.
About the Organizations Mentioned
Ionis_Pharmaceuticals
Ionis Pharmaceuticals is a pioneering biotechnology company specializing in **antisense therapy**, RNA interference, and CRISPR therapeutics. Founded in 1989 and based in Carlsbad, California, Ionis has been at the forefront of RNA-targeted drug development, aiming to treat serious diseases by selectively inhibiting the production of disease-causing proteins[2][3]. Originally named Isis Pharmaceuticals, the company rebranded to Ionis in 2015 to avoid association with the terrorist group ISIS. Ionis’ breakthrough came with the development of mipomersen, branded as Kynamro, a cholesterol-lowering drug approved by the FDA in 2013 after a complex partnership with Genzyme. This marked Ionis’ first major commercial success, providing significant revenue and validating its antisense technology platform[2][3]. Ionis has a robust pipeline with over two dozen drugs under development, focusing on cardiovascular, neurological, and rare diseases. Its technological advances include the use of constrained ethyl (cET) chemistry for enhanced drug potency and GalNAc conjugation, which improves liver-targeted delivery of therapies[3]. The company also founded Akcea Therapeutics, its commercial affiliate that markets drugs like TEGSEDI and WAYLIVRA in Europe and Brazil[3]. Key achievements include FDA approvals of multiple antisense therapies such as SPINRAZA (for spinal muscular atrophy), TEGSEDI, QALSODY, WAINUA, and TRYNGOLZA between 2016 and 2024, highlighting Ionis’ expanding impact in rare disease treatments[3]. In 2020, CEO Brett Monia refocused Ionis on advancing its wholly owned pipeline, emphasizing innovation and growth[3]. Ionis is recognized for its **bold scientific ambition**, lean decision-making culture, and strong commitment to corporate responsibility, including partnerships with patient advocacy groups and STEM education initiatives. This culture fosters rapid innovation and patient-centered development, positioning Ionis as
U.S. Regulators
The term “U.S. Regulators” broadly refers to the constellation of federal and state agencies, as well as self-regulatory organizations, that oversee the American financial system—a complex and dynamic ecosystem critical to both domestic and global markets. These regulators do not constitute a single organization, but rather a network of institutions with distinct, sometimes overlapping, mandates designed to ensure the stability, integrity, and fairness of the U.S. financial sector[4][6][8]. ## Core Functions and Structure U.S. financial regulators are responsible for creating and enforcing rules, licensing and supervising financial institutions, protecting consumers, and maintaining market confidence[3][7]. Major federal agencies include the **Federal Reserve System (the Fed)**, which manages monetary policy and supervises large banks and holding companies; the **Office of the Comptroller of the Currency (OCC)**, which oversees national banks; the **Federal Deposit Insurance Corporation (FDIC)**, which insures deposits and supervises state non-member banks; and the **Consumer Financial Protection Bureau (CFPB)**, which focuses on consumer rights in financial products[4][6]. The **Securities and Exchange Commission (SEC)** and the **Financial Industry Regulatory Authority (FINRA)** regulate securities markets and broker-dealers, with FINRA operating as a self-regulatory organization under SEC oversight[2][5]. ## Historical Context The modern U.S. regulatory framework emerged in response to financial crises. The Federal Reserve was established in 1913 after the Panic of 1907 to act as a lender of last resort and stabilize the banking system[1][4]. The Great Depression led to the creation of the SEC (1934) and FDIC (1933), while the 2008 financial crisis spurred the establishment of the CFPB under the Dodd-Frank Act (2010), expanding oversight to previously unregulated corners of finance[4][5]. ## Key Achievements U.S. regulators have