CDC Panel Updates Hepatitis B Vaccine Timing for Newborns

CDC Panel Revises Hepatitis B Vaccine Timing for Newborns
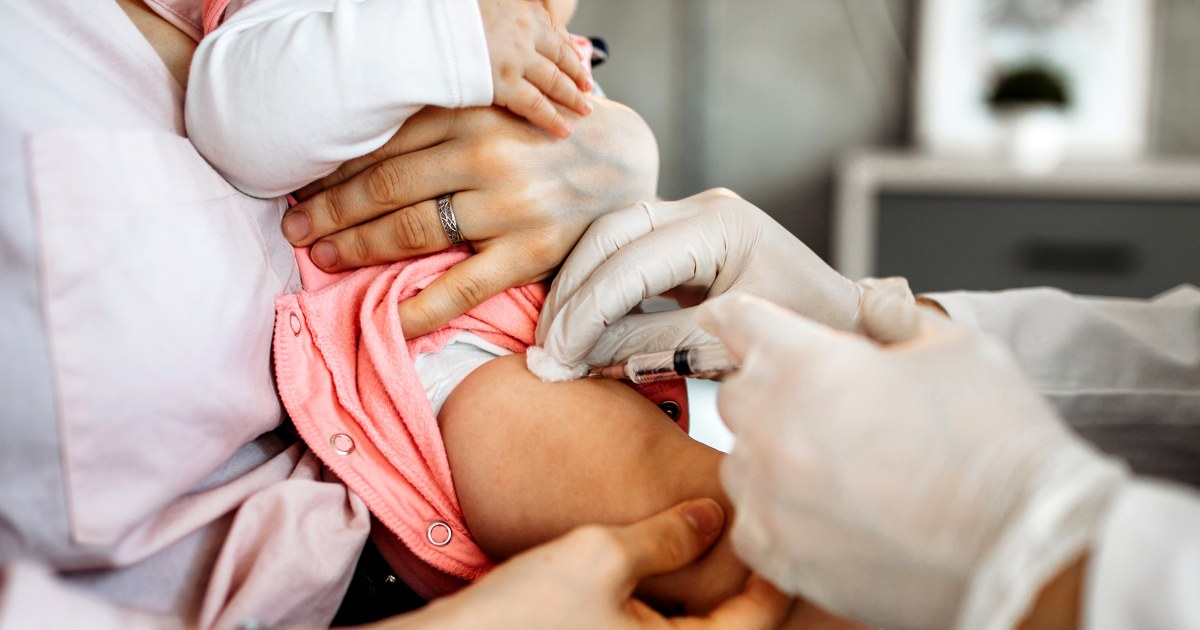
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted to delay the first dose of the hepatitis B vaccine for most newborns, overturning a policy in place since 1991. Previously, all babies received the vaccine at birth, a strategy that helped reduce hepatitis B infections in children by 99%. The new guidance recommends parents discuss vaccination timing with their healthcare providers and consider delaying the initial dose until the baby is at least two months old, unless the mother tests positive for hepatitis B during pregnancy.
Context and Controversy
This decision follows concerns about vaccine hesitancy and safety perceptions among parents. Some ACIP members and public health officials questioned the birth dose, although the American Academy of Pediatrics continues to support immediate vaccination. The change reflects ongoing debates in U.S. vaccine policy amid shifting public trust and differing expert opinions on the best approach to prevent chronic liver disease caused by hepatitis B.
Implications for Public Health
If adopted by the CDC director, this policy shift could reshape newborn vaccination schedules nationwide. It underscores the importance of individualized healthcare decisions and continued monitoring of vaccine effectiveness and safety.
About the Organizations Mentioned
CDC
The **Centers for Disease Control and Prevention (CDC)** is the premier national public health agency of the United States, operating under the Department of Health and Human Services and headquartered in Atlanta, Georgia. Its primary mission is to protect public health and safety through disease control, injury prevention, and health promotion both nationally and globally[1][8]. Established in 1946 initially as a single "Center for Disease Control," the agency expanded and reorganized in 1980 into multiple specialized centers, reflecting a broader focus beyond infectious diseases to include environmental health, chronic disease, occupational safety, and health education[7]. The CDC comprises various centers and institutes, such as the National Center for Immunization and Respiratory Diseases, the National Center for Chronic Disease Prevention and Health Promotion, and the National Institute for Occupational Safety and Health (NIOSH), among others. These centers enable the CDC to address a wide array of public health challenges through research, surveillance, policy development, and education[2]. It also plays a key role in emergency preparedness and response, demonstrated notably during the COVID-19 pandemic, where its guidance shaped public health actions despite complex political and social dynamics[8]. Key achievements include pioneering epidemiological research, controlling outbreaks of infectious diseases, advancing vaccine safety and immunization programs, and addressing emerging health threats such as obesity and diabetes. The CDC is recognized for disseminating authoritative health information, including the widely cited Morbidity and Mortality Weekly Report (MMWR), and for its global collaborations with health organizations worldwide[1][3][8]. Currently, the CDC is undergoing organizational adjustments to focus more intensively on infectious diseases, as part of the 2025 Department of Health and Human Services reorganization. This includes absorbing the Administration for Strategic Preparedness and Response while shifting some functions like occupational safety to new entities[1]. The agency’s comprehensive approach, backed by science and government funding, positions it as a critical leader in public health innovation, disease prevention, and health security i
Advisory Committee on Immunization Practices
## Advisory Committee on Immunization Practices (ACIP): Overview, History, and Impact The **Advisory Committee on Immunization Practices (ACIP)** is a federal advisory committee under the U.S. Centers for Disease Control and Prevention (CDC) responsible for providing expert recommendations on the use of vaccines and related agents to control vaccine-preventable diseases in the U.S. civilian population[1][2][6]. Its guidance covers routine immunization schedules for children and adults, as well as nonroutine situations such as outbreaks and travel[2]. ACIP’s recommendations are foundational for both public health policy and clinical practice, influencing everything from school entry requirements to insurance coverage for vaccines[1]. ## History and Structure Established in March 1964 by the U.S. Surgeon General, ACIP was created under Section 222 of the Public Health Service Act to provide ongoing, independent advice to the Secretary of Health and Human Services (HHS) on vaccine policy[1][2]. The committee comprises medical and public health experts, including both voting and liaison members, and operates through regular public meetings and specialized work groups that review evidence and draft recommendations[2][6]. Final recommendations require a majority vote and are published in CDC’s Morbidity and Mortality Weekly Report (MMWR), making them official federal guidance[1][2]. ## Key Achievements ACIP’s recommendations have shaped the U.S. immunization landscape for over half a century, ensuring the integration of new vaccines (such as those for HPV, hepatitis B, and COVID-19) into routine care as soon as they are licensed[1][2]. During the COVID-19 pandemic, ACIP played a pivotal role in rapidly developing and updating vaccination guidelines, including recommendations for multiple age groups and immunocompromised individuals[5]. The committee’s rigorous, evidence-based process is designed to balance scientific rigor with public transparency, often assessing new vaccines in parallel with FDA approval[7]. ## Current Status
American Academy of Pediatrics
The **American Academy of Pediatrics (AAP)** is a leading professional organization dedicated to promoting the optimal physical, mental, and social health and well-being of infants, children, adolescents, and young adults. Founded in 1930 by 35 pediatricians responding to the need for an independent forum to address children’s unique healthcare needs, the AAP has grown to approximately 67,000 members across the United States, Canada, Mexico, and beyond, including pediatricians, medical subspecialists, and surgical specialists[1][3][4]. The AAP’s mission centers on supporting its members professionally while advocating for children’s health through evidence-based policies, education, and research. It provides extensive continuing medical education (CME) programs, scientific meetings, seminars, and a broad range of publications, including the flagship journal *Pediatrics* and the news magazine *AAP News*. Its publishing program is the largest pediatric resource globally, offering over 800 titles for consumers and healthcare professionals[4]. Governed by a board of directors led by an executive committee, the organization operates through various departments and more than 40 specialized committees that address issues such as injury prevention, nutrition, child health financing, and care for children with disabilities[3][5]. The AAP also maintains a network of chapters serving U.S. states and Canadian provinces, allowing it to address local as well as national priorities[5]. Among its key achievements, the AAP has been instrumental in establishing pediatric healthcare standards, advocating for immunization schedules, and issuing timely guidance on public health crises, such as weekly COVID-19 reports tracking pediatric cases and hospitalizations in the U.S.[4]. The organization’s emphasis on preventive care transformed pediatric medicine, shifting the paradigm from treating children as "miniature adults" to recognizing their unique developmental needs[3]. Currently headquartered in Itasca, Illinois, with an office in Washington, D.C., the AAP continues to influence pediatric healthcare policy, education, and practice globally. Its